The Basis of Body Weight Change ...
-
How does body weight change?
-
What is the basis of weight loss or gain?
-
How do we know how much energy is in food?
-
Do we generate the same amount of energy when using energy nutrients in our body as generated in a lab?
-
How are energy nutrients used by our cells?
-
What is anaerobic energy metabolism?
-
What is aerobic energy metabolism?
-
What are the by-products of energy metabolism?
-
What processes use fat for energy?
-
How are amino acids broken down?

How does body weight change?
Body weight changes as components of body composition change. That means that a loss of body fat would decrease weight and a gain of body fat would increase it. However it is important to realize that gains in body fat are often accompanies by minor changes in supportive tissue such as muscle, skin and bone. The same can be said of muscle. Changes in muscle mass can result in minor changes in bone, skin and blood mass as well. For most people body weight will change largely due to alterations in either or both body fat and muscle. For regular exercisers and athletes, muscle mass becomes a significant consideration in understanding how much they weigh. Meanwhile, for most people though, the scale goes up as body fat is accumulated. In either case, changes in body weight will depend on their energy (calorie) balance. In addition, reduced physical activity, which often happens during adulthood, can reduce muscle mass and theoretically lower body weight. However, what’s more typical is that losses in muscle are paralleled by gains in fat tissue which counterbalances the weight loss or can lead to weight gain is the accumulation of fat exceeds loss of muscle.
What is the basis of weight loss or gain?
The basis for weight loss and weight gain is energy or calorie balance. To an economist, it would be a simple model of supply and demand; for us, it allows us to use those algebra skills we developed in high school. If the calories contained in the food we eat (supply or positive) exceeds the calories expended (“burned”) by our body (demand or negative), then we will store the surplus. Quantifying the energy content of foods is easy. We can simply read the food label or look at a Calorie chart. A food’s energy content is the sum total of the energy contributions of its protein, carbohydrate, fat, and alcohol. However, quantifying the energy that we expend over the course of a single day and assessing how our energy expenditure may fluctuate over time with respect to different situations is a bit more complicated.
How do we know how much energy is in food?
When scientists want to know the energy content of a food, they can place the food item in an insulated chamber, called a bomb calorimeter, and “combust” it. Combustion requires oxygen (O2) and the products of combusting foods in a bomb calorimeter include CO2, water and heat. In addition, if the food contains protein or amino acids, some nitrogen-containing gases will also be produced. Since heat energy is typically measured in calories1 it is applied to food energy and the energy used in our body. In separate experiments, scientists can also determine the individual amounts of carbohydrate, protein, fat, and alcohol in a given food. The approximate energy equivalent of 1 g of these substances is as follows:
1 gram of carbohydrate = 4 calories
1 gram of protein = 4 calories
1 gram of alcohol = 7 calories
1 gram of fat = 9 calories
If we were to add up the energy contribution of the individual energy nutrients in a food, it should approximate the total calories of heat measured in a lab (e.g. bomb calorimeter).
Do we generate the same amount of energy when using energy nutrients in our body as generated in a lab?
We combust energy nutrients in our cells and in the process generate the same amount of energy as in the lab. In fact, the reason we bring O2 into our body is so that it can be used in the combustion of energy nutrients within our cells. Furthermore, CO2 is produced during the combustion of these energy nutrients in our cells and we must breathe it out. Despite several similarities between the combustion of energy nutrients in a bomb calorimeter and in our cells there are a couple of fundamental differences. First, when amino acids and proteins are combusted in a bomb calorimeter, nitrogen-containing gases are produced. Contrarily, when amino acids are used for energy in our cells, most of the nitrogen is ultimately used to make urea. Second, the combustion of energy nutrients in a bomb calorimeter is for the most part an instantaneous process, while the combustion of energy nutrients occurring within our cells happens over a series of many chemical reactions (energy pathways). Last, unlike a bomb calorimeter, when we combust energy nutrients in our cells, we capture roughly 40 percent of the energy released in the formation of ATP. Meanwhile, the remainder of the energy released in the breakdown of energy nutrients is converted to heat and must be released from our body.
How are energy nutrients used by our cells?
Carbohydrates, amino acids, fat, and alcohol can all be used by our cells to make ATP. Although the specific energy pathways involved in the metabolism of these substances are somewhat unique, they are indeed interconnected at various points. This allows us to convert glucose and certain amino acids to fatty acids and also to convert amino acids, glycerol, and lactate to glucose. However, only certain tissue will engage in these conversion activities.
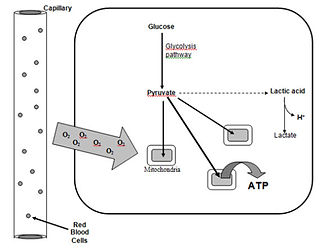
What is anaerobic energy metabolism?
Energy pathways in our cells occur in either the mitochondria or the intracellular fluid (cytoplasm). In the latter monosaccharides like glucose become engaged in an energy pathway called glycolysis. All cells can use glucose for energy; meanwhile fructose and galactose are used by the liver mainly. Glycolysis his pathway converts glucose to two molecules of pyruvate. In this process, two ATP and heat energy will be generated. Since these ATP will be generated without the need for O2, glycolysis is often referred to as anaerobic energy metabolism. Pyruvate has several options, depending on the type of cell and what is going on inside of that cell. If the cell lacks mitochondria, such as in RBCs, pyruvate is converted to lactic acid (lactate). This lactate enters the blood and can serve as fuel for certain other organs such as the kidneys. Meanwhile, astrocytes that create the blood-brain barrier, which separate the blood from the cererbral spinal fluid bathing the nourishes the brain and spine, generate lactate which neurons in our brain can use. Perhaps the most famous source of lactate acid is by muscle during intense exercise such as weight lifting or sprinting.
What is aerobic energy metabolism?
In order for pyruvate and lactate from glycolysis or fatty acids and amino acids to be used for energy in cells there need to be two things - mitochondria and ample O2. Because the need for oxygen energy generation in mitochondria is called aerobic. In most cells the pyruvate generated by glycolysis enters mitochondria for combustion. In addition, cells in certain tissue such as kidneys, liver, brain and muscle will convert lactate back to pyruvate which can enter the mitochondria or the lactate can enter mitochondria directly. Meanwhile some amino acids are converted to pyruvate as well or enter mitochondria directly like fatty acids.
Once inside the mitochondria, pyruvate can be converted to another molecule called acetyl CoA. Acetyl CoA can then enter another energy pathway called the Kreb’s cycle. During several of the chemical reactions that take place in our mitochondria, electrons are removed by carrier molecules and transported to special links of proteins embedded in the inner membrane of mitochondria. These special links of protein are called the electron-transport chain. The electrons are passed from the carrier molecules to the electron-transport chain and then, like a bucket brigade, are passed along its length. As electrons are passed along the electron-transport chain, energy is released which drives the formation of ATP. Each of our mitochondria probably contains thousands of electron-transport chains. Oxygen (O2) is needed to receive the electrons reaching the end of the electron-transport chain. Subsequently, the oxygen and electrons are coupled with hydrogen to make H2O. This serves to generate a little water in our body on a daily basis.
What are the by-products of energy metabolism?
When energy nutrients are combusted completely by aerobic processes, the end products will be CO2, H2O, ATP, and heat. The CO2 is actually a product of several reactions in our mitochondria. Since the need for CO2 is somewhat limited in our body, it is considered a waste product and must be removed by our lungs. If O2 is absent from a cell, the electron-transport chain will become jammed up with electrons and stop functioning. At this point that cell will have to rely more heavily upon anaerobic ATP generation. This is perhaps most obvious in skeletal muscle during heavy exercise. The increased reliance on anaerobic energy metabolism in skeletal muscle leads to the production of more and more lactic acid.
What processes use fat for energy?
When we use fat (triglyceride) for energy, both the fatty acid and glycerol can be used in energy pathways. Fatty acids enter an energy pathway called beta-oxidation which takes place within the mitochondria. Beta-oxidation produces several molecules of acetyl CoA, which can then enter the Kreb’s cycle. Also during beta-oxidation electrons are removed and transported to the electron-transport chain by the special carriers. Therefore, fatty acids require mitochondria and O2 in order to be used for energy; they are completely aerobic. Meanwhile, glycerol’s importance, from an energy standpoint, lies mainly in its ability to be converted to glucose in the liver during fasting or exercise.
How are amino acids broken down?
Amino acids can be used for ATP production in several ways. By consuming a lot of protein, excessive amino acids will be broken down in the liver. Once the nitrogen is removed from the amino acids, the remaining molecule can be converted to molecules in the energy pathways such as pyruvate, acetyl CoA, or those that are part of the Kreb’s cycle. This makes the generation of energy from amino acids aerobic. Meanwhile, during fasting and endurance exercise some amino acids can be converted to glucose in the liver. And, some amino acids can be used during fasting to produce ketone bodies. Both the glucose and ketone bodies produced via amino acids will be used by other tissue such as the brain and muscle. .